Techincal Data
Food polysaccharides can be classified simply as thickeners or gelling agents. In most cases, they are used in foods to impart stability during transport and storage, and desirable sensory qualities in some instance, they are used primarily as processing aids.
Although many polysaccharide thickeners are commercially available selection of the best candidate for a particular application is usually relatively easy and only requires a knowledge of the rheological properties of the polysaccharide in solution. Such knowledge is readily available in textbooks (e.g. Refs 1 and 2 ) and numerous publications in the scientific literature or from the polysaccharide suppliers. It is true to say that in some applications different thickeners may be used interchangeably from a functional standpoint and in these situations, the ultimate choice is usually determined by relative costs. In many cases, however, cost considerations are secondary to the subtle rheological benefits one material may provide relative to another.
The situation is somewhat different with hydrocolloid gelling agents. The term hydrocolloid rather than polysaccharide is used here because gelatine, one of the most important gelling agents, is a protein of animal origin. The important gelling polysaccharides are agar, high- and low- methoxy pectin carrageenan, alginate, starch and the mixed gelling system, carrageenan / locust-bean gum. Furcellaran, xanthan gum / locust-bean gum, some cellulose ethers and konjak mannan are also used commercially to produce gels but are of lesser importance, at least on a world-wide basis.
US market for gelling hydrocolloids has recently been estimated at 20.5 million kilograms excluding starch. Inclusion of starch gives a figure of around 84 million kilograms but this is somewhat misleading because it includes applications in which the starch used at relatively high concentrations, functions as a thickener rather than a true gelling agent. As in the case of polysaccharide thickeners, theological considerations, especially in the case of starches, are important when using these gelling agents. However, other properties also assume major importance. These properties include melting and setting temperatures, setting rates, clarity, purity and suitability for in-line processing.
In molecular terms, gels are meta-stable, three-dimensional polymer networks formed by chemically or thermally-induced interchain association. This intrinsic instability can lead to syneresis on storage. Syneresis may also be pronounced when the network is subjected to extreme variations in temperature such as freezing and thawing. Syneresis tends to be more of a problem in gelled than in non-gelled systems because of the reduced mobility of the polysaccharide chains. Gelling agents are generally not interchangeable, since each provides its own characteristic gel texture. For example, starch jelly candies, as the name implies, can only derive their unique texture from retrogradation of specific starches. While the standard for dessert gels is the gelatine jelly.
Each of the above gelling systems has been discussed in detail in other chapters of this book. However, a few additional comments are appropriate. In order to use these systems effectively, specialist knowledge is required. This is particularly true for the ion-sensitive materials, carrageenan, alginate and pectin. Despite the fact that they have been researched extensively and the general principles governing their use are readily available, their effective utilization still often relies heavily upon technical input from the suppliers. Gelatine and agar are simpler to use but again. Inadequate knowledge of their chemistry and properties and the differences between the various grades available commercially can lead to problems. Starch is by far the most widely used food hydrocolloid but surprisingly, our state of knowledge of starches in their various natural and modified forms in no way reflects their importance. Thus, selection of a starch for a specific application is often made without the proper technical considerations. Because of the abundance of starch, supply is not a problem. However, the chemical modifications required to give starches the desired in-use characteristics in many applications is giving rise to increasing cause for concern. The supply situation for the other gelling hydrocolloids is much less certain. Seaweed, the source of alginate, Carrageenan, furcellaran and agar is subject to the vagaries of nature and is not always available in sufficient quantities to satisfy market demand which can lead to hydrocolloid shortages. The scarcity and high price of agar have severely curtailed us usage in foods. Today, manufactures of gelling agents from seaweed obtain their raw materials from areas all over the world. Political instability in some of these areas gives rise to concern for the continued availability of these materials.
Over the past 20 years, bacteria have become an increasingly important source of polysaccharides. Dextran, a blood plasma extender and a key constituent of certain types of chromatographic materials, and xanthan gum, the most extensively tested and one of the most intensively researched food polysaccharides, are good examples of bacterial polysaccharides. Kelco Division of Merck & Co.Inc. has pioneered the development of bacterial polysaccharides and has now isolated in excess of 900 gum-forming bacteria. Although all of the gums produced by these bacteria are of scientific interest, most are not of commercial value because they do not offer significant advantages over existing alternatives. However, there are a few notable exceptions, namely rhamsan gum (S-194), a more efficient suspending agent than xanthan gum; Biozan welan gum ( S-130), a thickener for oil-field applications with superior thermal stability to xanthan gum and gellan gum ( S-60), a novel gelling polysaccharide.
Gellan gum offers a potential solution to many of the problems that exist with current gelling agents. Being a fermentation product, it can be produced on demand and with consistent quality. Availability and variability are, thus, not concerns. It is functional at very low use levels and is therefore, very efficient in many applications. Gellan gum can provide a range of gel textures as opposed to a single characteristic texture. Consequently, it can be used to mimic the texture of existing gelling agents or to create new textures. In today’s food industry, a tool to create new textures and hence permit the creation of new , differentiated food products would be highly desirable. Gellan gum is not a difficult product to use and although it is anticipated that eventually various form for a particular application will be determined by textural rather than by complex functional considerations. It should be stressed that gellan gum is in its early development stage, and much still has to be learned about its basic chemistry, properties, applications and , indeed, its ultimate utility in the market place. In this respect, it is encouraging that, for the first time in the history of hydrocolloids, commercial development is proceeding in parallel with the generation of fundamental scientific data on the product. The state of the art on gellan gum is discussed in detail in the following sections.
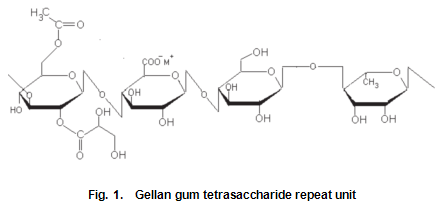
The monosaccharide building units of gellan gum are glucose, glucuronic acid and rhamnose in the molar ratios of 2:1:1. The primary structure, a tetrasaccharide repeating unit is shown in Fig.1. In the native form of the polysaccharide, there are approximately one and a half O-acyl groups per repeating unit. Originally the O-acyl substituent was thought to be O-acetyl, resulting in the various forms of gellan gum being referred to as high-and low-acetyl, and so on. Recent studies by Kuo et al. suggest that gellan gum contains both O-acetyl and O-L-glyceryl substituents on the 3-linked glucose unit, the former tentatively assigned to the 6-position and the latter to the 2-position. Analysis of samples in our laboratory following this work has indicated that glycerate substitution predominates over acetate. Undoubtedly, these bulky glycerate groups hinder chain association and account for the significant changes in gel texture that accompany deacylation. Inclusion of bulkier substituents on the gellan gum backbone has an
even more dramatic impact on properties. For example, welan gum and rhamsan gum, which have the same backbone as gellan gum but are substituted by mono- and disaccharide side-chains, respectively, have no similarity to gellan gum in solution behavior.
The shape or conformation adopted by the gellan gum molecule as a result of this primary structure has been under investigation for a number of years using X-ray crystallography. The quality of early diffraction patterns was not adequate to permit a detailed structural analysis. Subsequent work at Bristol, although resulting in high-quality diffraction patterns from well-oriented polycrystalline samples, also failed to produce a structure consistent with the X-ray data. A recent re-examination of the Bristol data has indicated that gellan gum forms an extended intertwined, three-fold, left-handed parallel double helix. Molecular shape in the solid state is usually an indicator of molecules associate in solution. The mechanism whereby gellan gum molecules associate in solution is believed to involve ion-mediated aggregation of double helices.
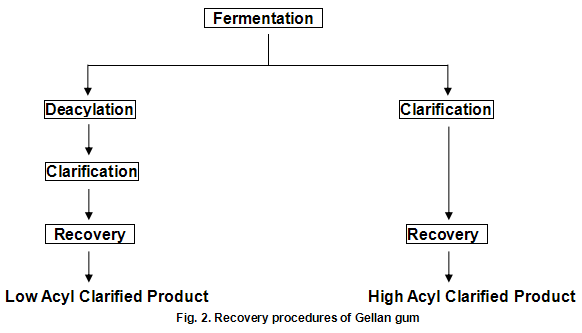
Gellan gum is produced by the bacterium Pseudomonas elodea. The gum is formed by inoculating a carefully formulated fermentation medium with this organism. The medium consists of a carbon source such as glucose, a nitrogen source and a number of inorganic salts. The fermentation is allowed to proceed under sterile conditions with strict control of aeration, agitation, temperature and pH. When fermentation is complete, the viscous broth is pasteurized to kill the viable cells and then subsequently processed to recover the polysaccharide in either the fully acylated native form or the deacylated form, as show in Fig.2. Gelrite-gellan gum for microbiological media and related applications and Kelcogel food grade gellan gum ---are low acyl products. Gels from the native material can loosely be described as cohesive and elastic, while those from the deacylated materials are strong and brittle. Materials of intermediate acyl content, which can be obtained by careful control of the deacylation step, provide gel textures intermediate between those of the native and fully deacylated products.
4.a Solubility
To date, most of the studies on gellan gum have focused on the low-acyl materials. These are produced as mixed salts, predominantly in the potassium form but also containing divalent ions such as calcium. Typical levels of the major cations in Gelrite are: Ca2+, 0.75%; Mg2+,0.25%; Na+,0.70%; and K+,2.0%. Low-acyl gellan gum is only partially soluble in cold water. Solubility is increased by reducing the ionic content of the water and by conversion of the gum to the pure monovalent salt forms, but complete solubility of Gelrite is only achieved in deionized water using the pure monovalent salt forms. Low-acyl gellan gum is dissolved by heating aqueous dispersions to at least above 70℃. Progressively higher temperatures are required as the ionic strength of the aqueous phase is increased. Except in the case of Gelrite at low concentrations in the absence of ions, subsequent cooling of the hot solutions always results in gel formation. Gels can be formed with Gelrite in concentrations as low as 0.05%. Suppression of solubility by the inclusion of ions is a useful tool for the practical utilization of low-acyl gellan gum. In this way, the gum can be easily pre-dispersed in water without encountering hydration problems, and can be activated simply by heating. Use of gellan gum in this manner is analogous to the use of native starches, which, being cold-water-insoluble granules, can be conveniently slurried in water prior to cooking. Solutions of gellan gum will react in the cold with mono and divalent ions to form gels and, depending on the types and levels of ions, the resulting gels may not melt on heating. To circumvent this usually undesirable situation, it is recommended that, in applications where partial or complete pre-solution of gellan gum is unavoidable, the gellan gum be incorporated above 70℃. Bearing in mind the above considerations, there are a number of alternative ways of incorporating low-acyl gellan gum into a given system. It may be added alone or in combination with other dry or liquid ingredients to a cold mix that is then heated and cooled to induce gelation. Alternatively, it may be added to a mix that has been pre-heated above 70℃. The preferred method of addition is best determined by consideration of the ingredients in the formulation and processing conditions. The ions present in the system have a major impact on the quality of the final gel and for best results ions additional to those inherently present in the system may be required. These can also be added in the cols or after heating.
4.b Rhcology of Solutions
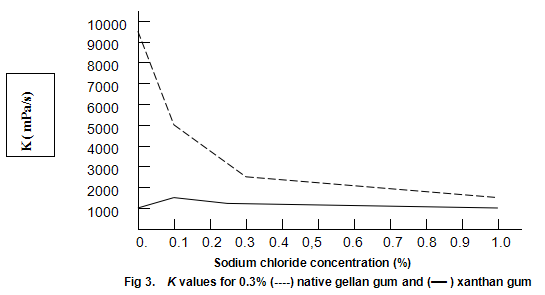
Native gellan gum on heating and cooling in the presence of cations forms cohesive, elastic gels similar to those obtained by heating and cooling mixtures of xanthan gum and locust-bean gum. Since this texture dose not appeal to most consumers, native gellan gum alone is not expected to see widespread utility as a gelling agent. However, when dispersed in cold water, it provides extremely high viscosities. A possible limitation to its use as a thickener is high sensitivity to salt. This effect is shown in Fig.3, which compares the viscosities of 0.3% solutions of xanthan gum and native gellan gum at different concentrations of salt. The viscosities recorded are K values derived from the ‘power-law’ equation, η=Kγ n-1, and are approximations of the viscosities at one reciprocal second. The well-known stability of xanthan gum viscosity to changes in salt concentration is apparent. In contrast, the viscosity of the native gellan gum displays a strong dependence on salt concentration. The native gellan gum solutions are highly thixotropic and the apparent high viscosities appear to be the result of the formation of a gel-like network. Similar thixotropic behaviour is observed when low concentrations of xanthan gum /locust-bean gum are dispersed in cold water.
5.a Measurement of Gel Texture
Before considering the influence of ions in greater detail, a discussion of gel texture and gel texture measurement is necessary. A wide array of terms, including elastic, brittle, hard, firm, cohesive and rubbery are , are used to describe the sensory attributes of gels. In contrast, objective measurement of texture is widely restricted to measurement of gel strength. This parameter is widely used as an indicator of the quality of a particular gelling agent and is obtained using a variety of instruments. Although providing a gel strength value, different instruments do not always measure the same textural parameter. A bloom gelometer, for example, provides an indication of the perceived firmness or modulus of a gel, while a Marine Colloids Gel Tester determines the force required to rupture the gel. Clearly, these parameters are not the same in textural terms. Gel strength measurements are thus only of limited value from a textural standpoint and, even when the conditions and instrument for measurement are specified, a full description of gel texture is not realized. For this reason, comparison of different gelling agents on basis of gel strength can lead to highly misleading conclusions. Only in cases where a comparison of the qualities of different grades of a specific gelling agent is required is gel strength of real value. Gel strength is normally used, for example, as an indicator of the quality, or Bloom strength of gelatine.
In the early 1960s, the problem of correlating objective measurements with the sensory perception of texture in foods was addressed by General Foods. The technique of texture profile analysis (TPA) that evolved from these studies has been further developed in our laboratory to permit routine measurement of gel texture.
As practiced in our laboratory, the technique involves preparing gel disc by allowing hot solutions to cool in plastic ring moulds. The gel disc, after removal from the mould, is placed on a plastic plate with a roughened surface and compressed by the Instron crosshead normally to 30% of its original height (70% compression), using a compression rate of 2 in/min. The crosshead is then withdrawn and the compression cycle is repeated. The textural parameters measured are shown on the idealized force deformation curve in Fig.4.
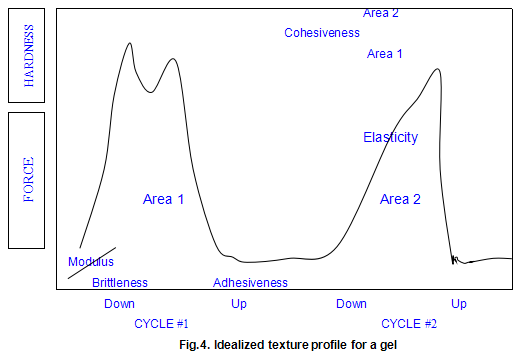
The modulus is the initial slope the force deformation curve .This is a measure of how the sample behaves when compressed a small amount .The modulus usually correlates very closely with a sensory perception of the sample’s firmness. The measurement is analogous to squeezing a piece of fruit to determine its ripeness. Modulus is expressed in units of force per unit area (usually in pounds force per square inch or newtons per square centimetre).
Hardness is defined as the maximum force that occurs at any time during the first cycle compression. It may occur when the gel initially breaks (as is shown in the ‘idealized’ plot) or it may occur later in the test as the sample is flattened and deformed. In most cases, the hardness is correlated to the rupture strength of the material. It is similar to gel strength measured on a Marine Colloids Gel Tester and is expressed in units of force (pounds force or newtons ).
The first significant drop in force-deformation curve during the first compression cycle is defined as the brittleness. It is the point of first fracture or cracking of the sample. A gel that fractures very early in the compression cycle is considered to be more brittle or fragile than one that breaks later Brittleness is measured as the percentage strain required to break later. Brittleness is measured as the percentage strain required to break the gel. As the number becomes smaller, it indicates a more brittle gel because the gel breaks at a lower strain level. Since the gel normally compressed to a 70% strain level, the maximum value for brittleness is 70%. Such a high value would represent a gel, such as xanthan gum / locust-bean gum, that is not brittle at all.
Following the first compression cycle, the force is removed from the sample as the Instron crosshead moves back to its original position. If the material is at all sticky or adhesive, the force becomes negative. The area of this negative peak is taken as a measure of the adhesiveness of the sample. Obviously, the more sticky of the product, the higher the adhesiveness value. There are no real units for this parameter. It is expressed in the internal integrator units of the computer.
As the second compression cycle is begun, the sample’s elasticity is determined. By noting where the force begins to increase during this second compression cycle, a measure of the sample height may be obtained. It the sample returned to its original height, the elasticity would be 100%. The elasticity is a measure of how much the original structure of the sample was broken down by the initial compression. In sensory terms, it can be thought of as how ‘rubbery ’the sample will feel in the mouth. The units are dimensionless (a length divided by another length) and are usually expressed as a percentage.
Cohesiveness is measured by taking the total work done on the sample during the second cycle and dividing it by the done during the first cycle. Work is measured as the area under the respective curves. This ratio is usually expressed as a percentage. Samples that are very cohesive will have high values and will be perceived as tough and difficult to break up in the mouth. As with elasticity, there are no units, since the quantity is dimensionless.
5.b Factors Affecting Gel Texture
Figure 5 shows the hardness and modulus of Gelrite (0.25%) as a function of calcium ion concentration at neutral pH. Gels were prepared by dispersing Gelrite in distilled water, heating to 70-75℃, adding the appropriate concentration of ions, and cooling. Although, in the absence of ions, only a very weak gel is formed, the hardness rapidly increases to a maximum at low Ca2+ levels and then gradually deceases as the ionic concentration is increased. The modulus shows a somewhat more symmetrical increase and decrease, and between 0.016% and 0.05% calcium (400 and 1250 ppm CaCO3) modulus values between 5.5 and 6.5 N/cm2 are obtained. Within this same range, the hardness varies between around 3.0 and 4.0 lb. Thus, provided products are formulated so that the concentration of free ions is in this range, modulus and hardness are fairly insensitive to changes in ionic composition brought about by variations in raw materials. It is noteworthy that since the bottom end of this range (400ppm CaCO3) is higher than the hardness encountered in most waters, some products may require the addition of ions for best results.
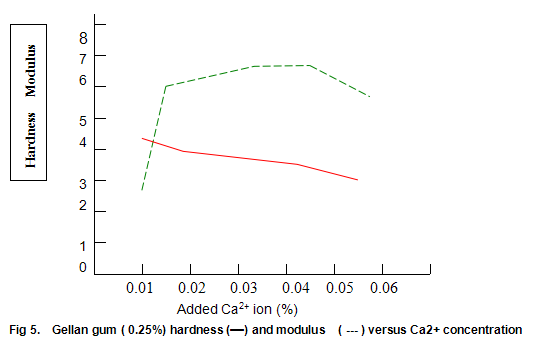
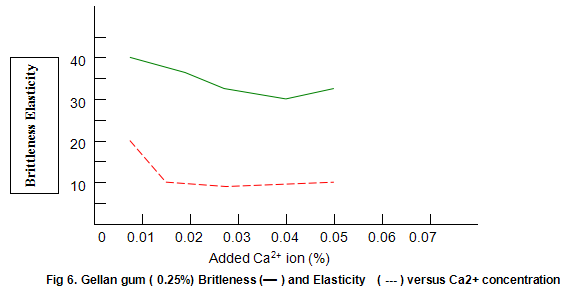
Figure 6 shows that Gelrite gels are brittle, with brittleness values between 30% and 40%. Some reduction in brittleness (higher values correspond to lower brittleness) is seen at lower ion concentration. In contrast, elasticity (Fig. 6) is highest under these conditions and drops rapidly as ion level is increased to an almost constant value of around 10%. Other divalent ions, notably Mg2+, have similar effects on gel texture. There trends are also observed with monovalent ions such as K+ and Na+. However, with these latter ions much higher concentrations are required. For example, maximum hardness requires an approximate 25-fold increase in the molar concentration of Na+ and K+ relative to Ca2+ or Mg2+.
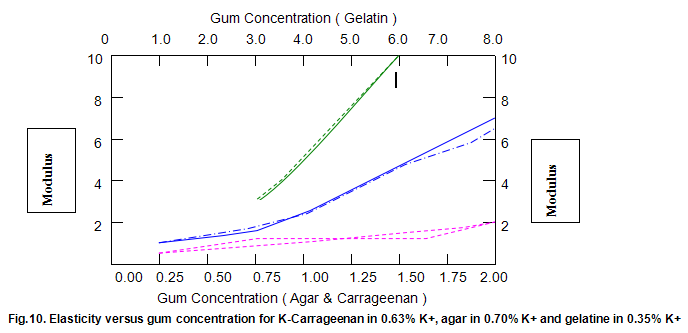
Comparative studies in our laboratory on the influence of ionic concentration on к-carrageenan (1.5%), agar (1.5%) and gelatine (5.2%) revealed, not unexpectedly, that the gel textural parameters for к-carrageenan were strongly dependent on K+ concentration. Potassium had less of an effect on gelatine texture and essentially no influence on agar texture. The level of potassium required to give the maximum modulus value for each of these hydrocolloids was then used in determining the relationship between modulus/hardness and gum concentration. The results are shown in Fig9 and 10. Figure7 compares the same parameters for Gelrite. As is indicated, Gelrite, at a given concentration, provides much higher hardness than the alternative hydrocolloids at the same use level.
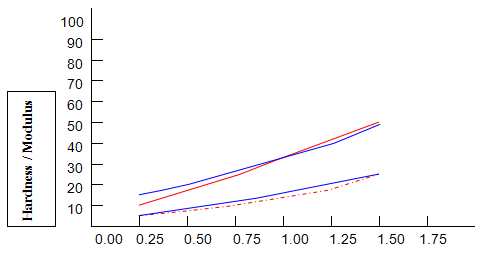
The difference is substantially more striking when the modulus are compared. Gel strength measurements based on hardness or modulus would thus clearly reflect the greater efficacy of gellan gum.figures7,9 and 10 also show the influence of pH on modulus and hardness.
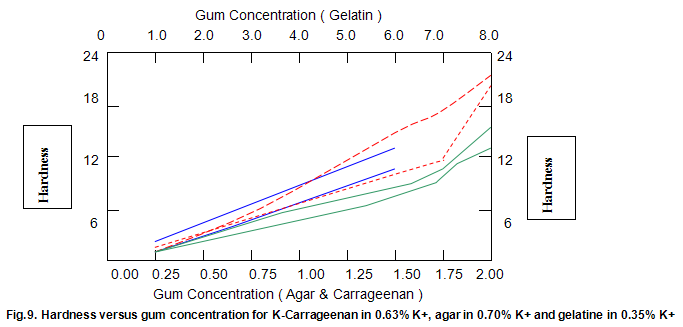
On reducing the pH to 4.0 by the addition of citric acid, no change in modulus is observed for all of the gelling agents. This is also true for gellan gum hardness and, at lower concentrations, for gelatine hardness. At higher concentrations of gelatine, lowering pH causes a reduction in hardness. Reduced hardness at pH4.0 is observed for all concentrations of k-carrageenan and agar, particularly in the case of the former.
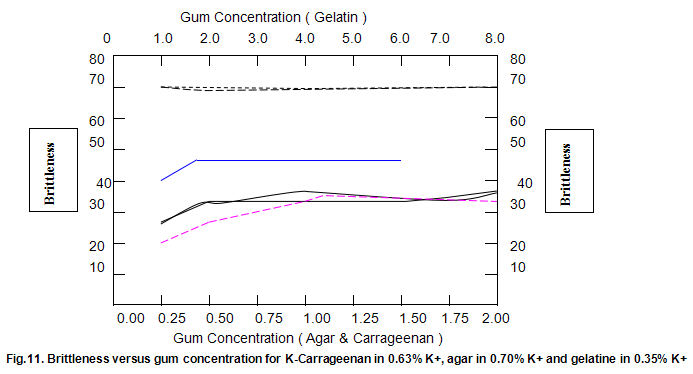
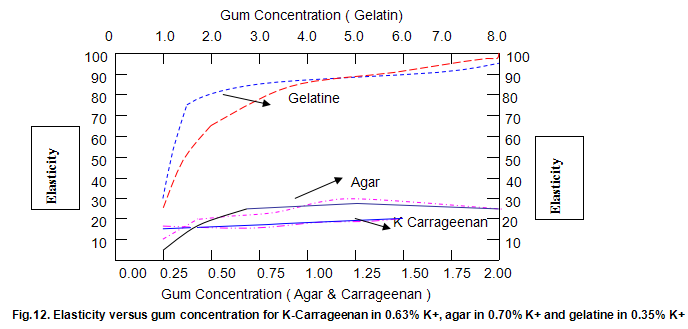
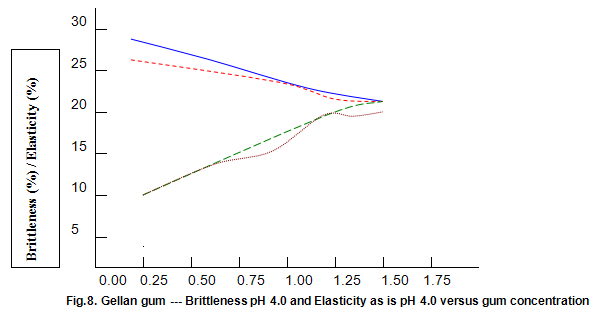
The dependence of brittleness / elasticity on Gelrite concentration is shown in Fig 8. The gels become slightly more brittle and elastic as concentration is increased. Brittleness of k-carrageenan, agar and gelatine remains almost constant above a threshold gum concentration, as shown in Fig.11. Except in the case of carrageenan, when a reduction in pH increases brittleness by around 10%, change in pH has little effect on brittleness. Figure 12 shows that the elasticity of k-carrageenan is largely independent of concentration and pH. The same figure indicates that pH has a minor influence on the elasticity values of agar and gelatine gels, which show a fairly rapid increase at low gum concentrations but tend to level off at higher concentrations.
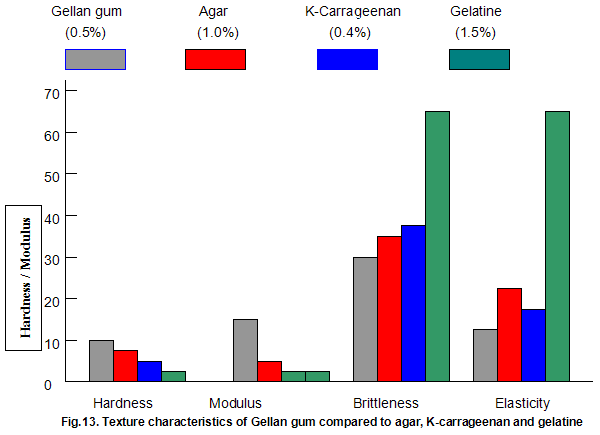
The textural characteristics of gels made from typical use levels of agar(1.0%), k-carragecnan(0.4%) and gelatine (1.5%) are compared to those of a 0.5% Gelrite gel in Fig.13. The data indicate that Gelrite gels can be qualitatively described as hard, firm, brittle and non-elastic, while a totally opposite description would be appropriate for gelatine. The fact that gelatine has a brittleness of 70% indicates that the gel did not break under the 70% maximum compression employed in the texture profile test procedure. Agar and k-carrageenan are slightly less brittle and more elastic than Gelrite but lower in hardness and, in particular, modulus.
5.c Melting and Setting Points
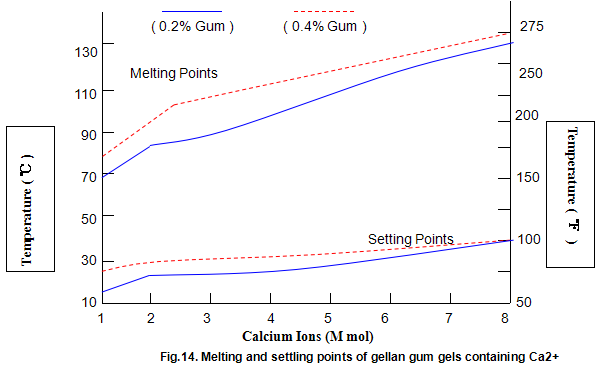
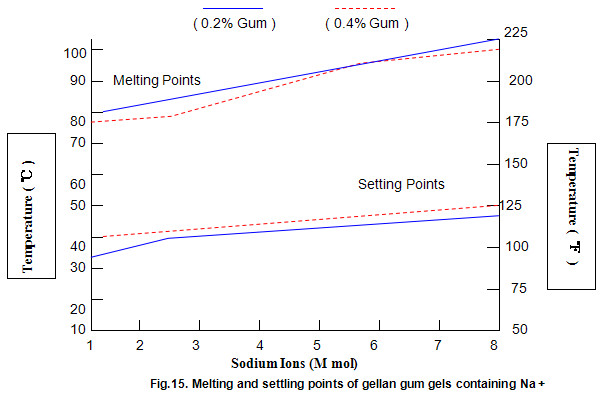
Key properties of gels are melting and setting points. For gellan gum, these strongly depend on ion concentration and type, and, to a lesser extent, on gum concentration, as shown in Figs 14 and 15. Most Ca2+ gels set between 25 and 40℃ while those with Na+ set in the range 40-50℃. Ionic concentration has a marked influence on melting temperature and at lower ion levels the gels re-melt on heating, while at higher levels the gels do not melt below 100℃. This latter property is useful in applications in which heat-stable gels are required.
5.d Comparison with other Hydrocolloids
Form the foregoing discussion, the similarity between gellan gum and agar and k-carrageenan is apparent. This similarity is true not only in textural terms but, for agar, in terms of the large hysteresis in setting and melting. The ion dependence of the properties of gellan gum and k-carrageenan is striking. Another similarity is the ability of cold solutions of both gellan gum and carrageenan to gel upon the addition of ions. Ion-induced gelation is a phenomenon more commonly associated with alginate and formation of alginate gels in the cold by the conrolled release of Ca2+ is well known and widely used industrially. There are indications that the
techniques used for preparing alginate gels may be appropriate for gellan gum in certain applications. Alginates also have a strong interaction with hydrogen ions and precipitation of sodium alginate form solution by conversion to the acid form through acid addition can be used in the manufacture of alginates. Likewise, acid precipitation is an effective and alternative means of isolating gellan gum. The gels produced form gellan gum by the addition of hydrogen ions are extremely strong. The fact that gelation of gellan gum is induced by ions both upon cooling like carrageenan and in the cold like alginate has led to its being described as the’ missing link’ in gelling hydrocolloids. The application of this statement is that understanding the molecular baisi of gellan gum gelation may help resolve the mechanism of carrageenan gelation, which is generally, although not universally, in contrast to alginate, where the accepted mechanism is ion-induced dimeric association of polymer chains in the cold.
5.e Blends with Other Hydrocolloids
Combinations of more than one hydrocolloid are widely used in foods. In fact, use of blends rather than a single hydrocolloid could be considered standard practice. Non-gelling hydrocolloids are normally used together to obtain optimal rheology. In some cases, xanthan gum guar gum being a good example, the combination is used to obtain a synergistic increase in viscosity. The changes in viscosity that result form blending non-gelling hydrocolloids can be predicted using the so-called log-mean blending law. Viscosity values that differ form those predicted indicate synergistic (greater than expected) or anti-synergistic behaviour. Combinations of gelling and non-gelling hydrocolloids or two or more gelling hydrocolloids are much more complex, and various possible network structures have been proposed. Since not a great deal is known about mixed polysaccharide gelling systems, the concept of synergism applied to these systems is generally in appropriate. Despite this lack of fundamental knowledge, mixed polysaccharide gels are well established commercially. Combinations of k-carrgeenan or agar and locust-bean gum are perhaps the best examples. The inclusion of locust-bean gum provides textural modification and allows reduction of the total polymer concentration required for gel formation. Use of the gelling system xanthan gum locust-bean gum to modify the textures of gels such as those form agar and carrageenan has also been suggested.
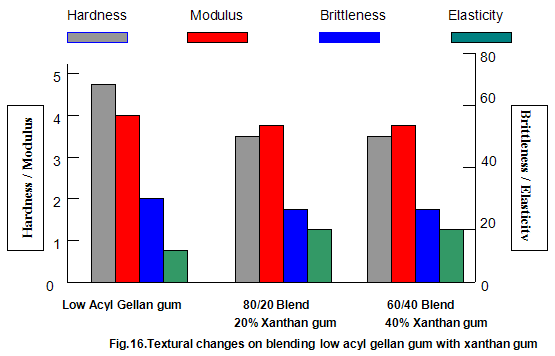
The effect of both gelling and non-gelling hydrocolloids on the texture of low-acyl gellan gum gels has been extensively studied. Commonly used thickeners such as guar gum, locust-bean gum, xanthan gum, carboxy-methylcellulose and tamarind gum, when added to gellan gum in progressively increasing amounts while maintaining a constant total gum concentration, cause a progressive reduction in hardness and modulus. Brittleness remains essentially constant, with an accompanying slight increase in elasticity. These effects are shown for low-acyl gellan gum/xanthan gum combinations in Fig.16. For the key textural parameters, hardness, modulus and brittleness, modulus and brittleness, these thickeners function essentially as inert diluents and the texture of the resulting blends is similar to the texture of low-acyl gellan gum alone at a concentration equivalent to that in the blend. It is common practice to include thickeners in gelled systems to reduce syneresis, improve freeze thaw stability and, in some cases, eliminate unfavorable interactions between ingredients. Thus, some products formulated with gellan gum also require the presence of thickener.
The textural similarity between gels form low-acyl gellan gum, k-carrgeenan and agar has already been mentioned. Blends of low-acyl gellan gum and agar(0.50% and 0.25% total gum concentration ) provide gels in 4mM Ca2+ that show a decrease in hardness and modulus as the blend becomes richer in the agar gum component, the decrease being more pronounced at the higher gum concentration. Brittleness and elasticity values remain virtually constant around 34% and 14%, respectively. Similar blends of k-carrgeenan and low-acyl gellan gum in 0.16mM K+ show a rapid drop in hardness(4.5 to 2) in going form 0.5% low-acyl gellan gum, k-carrageenan and then rises to around 4 for carrageenan alone at 0.5%. Modulus falls sharply form 4.6 in going form 0.5% low-acyl ggellan gum alone to the 80:20 blend and remains between 1.5 and 2 thereafter. At 0.25% total gum concentration the same trends are apparent but less pronounced. As in the case of the low-acyl / agar blends, the low-acyl / carrgeenan blends have a fairly constant brittleness and elasticity irrespective of blend composition. The values for these latter textural parameters are almost identical to those for the low-acyl / agar blends. These data indicate that the characteristic brittle texture of low-acyl gellan gum gels cannot be substantially changed by progressive substitution with other brittle gelling agents.
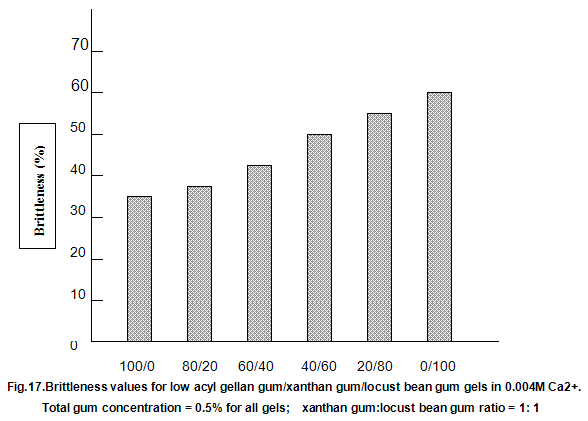
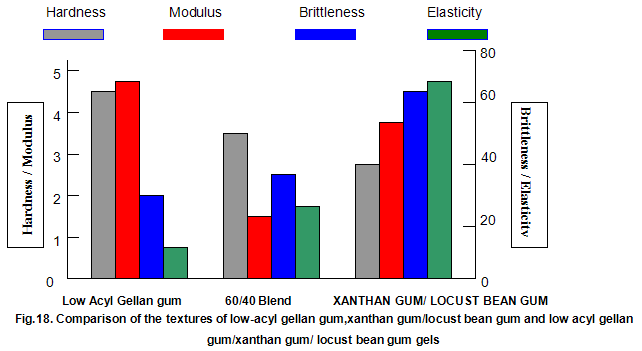
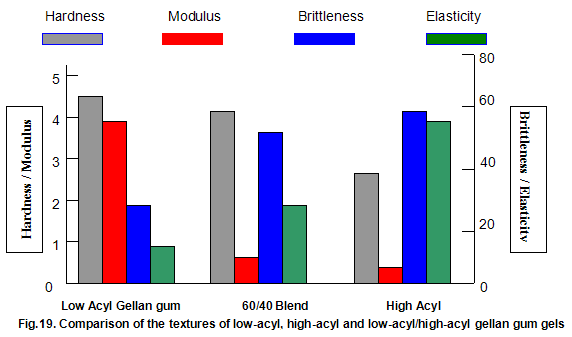
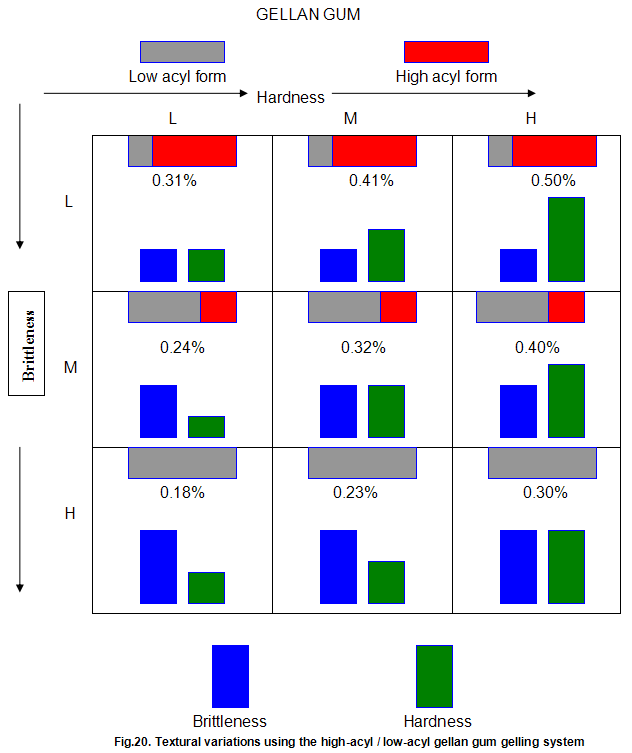
This is not the case when low-acyl gellan gum is used in combination with the xanthan gum / locust-bean gum gelling system. As can be seen in Fig,17, the xanthan gum gels become less brittle as the blend becomes richer in xanthan / locust-bean gum. Figure 18 indicates the other textural changes that take place. Hardness and modulus are reduced, while elasticity is increased. Similar textural changes are induced when locust-bean gum is replaced by other hydrocolloids, such as Cassia gum and konjak mannan , both of which are capable of interacting with xanthan gum to form gels with texture similar to those obtained form xanthan gum and locust-bean gum gels and native or high-acyl gellan gum is evident by comparing Figs18 and 19 . Consequently, it si perhaps not surprising, as indicated in Fig.19, that blends of high- and low-acyl gellan gum provide textural variations similar to those obtained by blending different ratios of low-acyl gellan gum and xanthan gum / locust-bean gum. For labeling purposes, achievement of textureal modification by blending gellan gum alone would clearly be referred. Figure 20 demonstrates the rang of different textures that can be obtained simply by using different proportions of the high-acyl form.
If starch is excluded, gelatine is the most widely used gelling agent. In contrast to the strong, brittle, non-elastic gels produced by low-acyl gellan gum, gelatine gels have a low modulus or perceived firmness and ggelatine offer another avenue to textural diversity. For example, addition of progressively increasing amounts of 250 Bloom type A gelatine to 0.25% low- acyl gellan gum, a typical in-use concentration, causes a gradual increase in hardness, modulus and elasticity and a gradual reduction in brittleness. Conversely, addition of low levels of low-acyl gellan gum, up to 0.05%, to replace up to around 1% gelatine in a 5% gelatine gel has no marked influence on the characteristic gelatine texture. It is conceivable, however, that the melting / setting temperatures of the gelatine may be advantageously increased by inclusion of low levels of the higher melting and setting low-acyl gellan gum. Low-acyl gellan gum / gelatine combinations may also help prevent toughening of gelatine gels upon refrigerated storage , allow
low-grade gelatins to be upgraded in quality or permit lower gum concentrations to be used in certain applications. A recent patent describes combinations of gelatine and different forms of deacylated gellan gum. In the context of gelatine, the excellent flavour release form low-acyl gellan gum gels is worthy of mention. This flavour release is a consequence of gellan gum’s ability to structure water in the gelled state at very low use levels rather than the gels having the ‘melt in the mouth’ characteristics associated with gelatine.
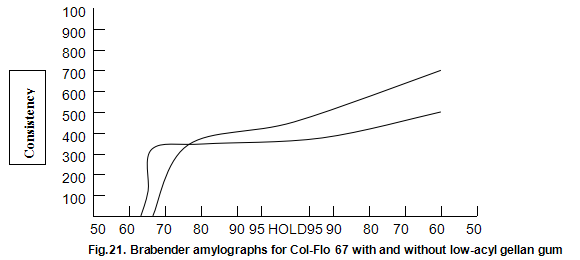
Raw and modified starches are often used to impart a characteristic heavy-bodied consistency and, in some cases, a gel-like structure to foods. Cold-water-dispersible instant starches are available but many starches require cooking to cause gelatinization and generate the desired functional properties. The molecular changes that occur when starch is cooked and cooled are still poorly understood. However, it is generally accepted that a cooked starch paste consists of swollen intact and ruptured granules within a continuous aqueous phase containing the solubilized amylase and amylopectin, the two component polysaccharides of starch. When starch is used, additional hydrocolloids are often required to modify texture, reduce syneresis and improve freeze thaw stability. Although possible mechanisms for the interaction between these hydrocolloids and starch have been suggested, current understanding is again poor. Consequently, starch hydrocolloid combinations are usually selected on an empirical basis. A standard indicator of performance of a starch system is the viscosity changes that occur during heating and cooling as measured on an amylograph. Amylograph data on the influence of low-acyl gellan gum on the modified starch, Col-Flo67 (National Starch and Chemical Corp.), are shown in Fig, 21. The gellan gum produces a more rapid increase in initial build-up of viscosity. Viscosity subsequently remains fairly constant on further cooking and then rises less rapidly than the viscosity of the Col-Flo 67 alone upon cooling. These limited results are difficult to interpret on a fundamental basis but suggest that starch gellan gum combinations are worthy of more detailed study. In practical terms, it has already been shown that in certain pudding and pie fillings the levels of modified starch can be reduced by a half by inclusion of around 0.1% Kelcogel gellan gum. In these products, the structure imparted by the gellan gum results in a fifmer shorter texture.
The compatibility of polysaccharides with proteins depends on a number of factors such as relative concentrations, pH, ionic strength, temperature time and, in case of foods, the nature of the other ingredients present. From a compatibility standpoint, model studies are thus of limited value and, as for starches, incompatibility problems that occur in products are most quickly solved by empiricism. Our experience with the interactions between gellan gum kk and proteins has also
been similar. Limited model studies indicated that while low-acyl gellan gum was compatible at neutral pH with milk proteins, soy, egg albumen, whey and sodium caseinate precipitation with all of these proteins occurred around pH 4. In contrast, it has been shown possible to produce a number of direct acidified and cultured dairy products using low-acyl gellan gum in combination with protective colloids such as guar gum and carboxymethylcellulose. Low-acyl gellan gum also shows good compatibility with proteins in non-acidified mile systems. The need to study protein / polysaccharide interactions under specific in-use conditions is emphasized by the fact that. Although low-acyl gellan gum and gelatine combinations are potentially useful as already discussed, precipitation can occur under certain conditions. The observation that sodium caseinate and soy protein can prevent gelation of low-acyl gellan gum without causing precipitation also requires further investigation to define more fully the conditions under which this occurs.
Gellan gum is a potential replacement for existing gelling hydrocolloids or, because of its unioue properties, a tool for the development of new products. Many applications have been identified and evaluated on a laboratory scale and some have already been successfully tested at the commercial level. However, much still remains to be done to establish the commercial utility of gellan gum, which can only be achieved by widespread exposure of the product to industry. To date, only a few prospective users have had first-hand experience with gellan gum.
The broad areas of application in which gellan gum has been tested are microbiological media, tissue-culture media, foods and pet foods. Selected industrial applications have also been cvaluated, namely deodorant gels, soft gelatine capsules, photographic films and microcapsules. Carrgeenan / locust-bean gum is commonly sued in room-deodorant gels. This system can be replaced with low-acyl gellan gum at around one-half to one-third the use level. Although low-acyl gellan gum alone provides a more brittle gel than the carrageenan / locust-bean gum gel, the texture produced by the latter can be simulated by inclusion of xanthan gum / locust-bean gum with the low-acyl gellan gum. When freeze thaw stability is required, propylene glycol may be included in the gellan gum gel, In photographic film and soft gelatine capsules blends of low-acyl gellan gum and gelatine have been shown to function as replacements for gelatine alone. Although further work is required to substantiate more fully the benefits of these blends, the fact that the soft felatine capsules do not dissolve in the gut when gellan gum is used is seen as an adbantage in certain situations. This property has led to the suggestion that gellan gum may be a suitable vehicle for controlled release of certain drugs. Microcapsules are usually formed by coacervation of gelatine for coacerbation and microencapsulation has been demonstrated in our laboratory and, more recently, by other studies.
The similarity between agar and Gelrite has led to the commercialization of Gelrite as an alternative to agar for microbiological media. Studies using 50 different bacterial species have shown that Gelrite can not only be substituted for agar in many routine media applications but can also give a higher degree of cell growth in certain situations. Gelrite is particularly useful for the culture of thermophilic microorganisms, as Gelrite gels are thermostable and can withstand prolonged incubations at high temperatures. In addition, acceptable gel strengths can be obtained using Gelrite at a lower level than agar and spreader colonies do not become too large. It has been demonstrated that Gelrite to agar for cultivation of mesophilic Methanobacterium organisms, the advantages being greater gel strength, reduced preparation time of plates drier media and, in the case of mesophilic Methanobacterium species, reduction of the extended incubation times required. In all of these microbiological media applications, the high purity of Gelrite and the water-like clarity of the resulting gels are distinct additional advantages. To maintain perspective, it should also be pointed out that a few problems have also been encountered in certain media when Gelrite is used. Some media are difficult to re-melt, the plates are sometimes more difficult to streak than are agar plates, and the high setting temperatures can cause hamolysis of blood for blood plates. Although failure to solve these probkems may prevent the use of Gelrite in some situations, the benefits obtained from Gelrite should lead to its establishment as a better alternative to agar for many microbiological media.
Agar is currently the gelling agent of choice for plant tissue culture. However, the presence of impurities or inhibitory factors, including sulphur, in agar can adversely affect growth. Gelrite offers a promising alternative to agar in his application because of its high purity. Recent studies on a limited number of plant species have shown that growth is indeed improved on media containing Gelrite. In addition, Gelrite could be used at one-fifth of the agar use level, showed good resistance to contamination by moulds, was easily washed form the plant tissue for transplantation, and allowed clear observation of root and tissue development. Despite these encouraging results, the full potential pf Gelrite for plant tissue culture requires a more extensive study involving a larger number of plants.
Pet foods are a major application for alginate and carrageenan. The typers of pet foods using these materials consist of comminuted meat bound in a gel matrix and are widely available in Europe and Australia, usually in canned form. In the United Kingdom and Australia, these products are also sold in non-sterile containers such as sausage casing and are sometimes referred to as dog-brawn. These brawns, which contain preservatives, have a short shelf life and are produced by a number of small manufactures supplying local markets. A desirable feature of the canned pet foods is the presence of meat-like chunks. These are sometimes produced in the cold by the reaction of alginate with calcium ions to produce a gel structure that provides structural integrity to the chunks during filling and in the early stages of retorting. Alternatively, the alginate may be used to bind the comminuted meat into a continuous gelled block. Again the gel is formed in the cold by standard techniques. Despite the advantage of cold make-up, use of alginate in pet foods is now largely restricted to the production of chunk and other shaped pieces. Low grade k-carrgeenan is now the product of choice for binding the meat in a gelled matrix. More specifically, blends of k-carrgeenan, locust-bean gum and potassium chloride are used, the carrageenan / locust-bean gum system providing a less brittle gel than carrageenan alone. Many varieties of pet food exist, each with its own particular blend of gelling agents carefully selected to provide the desired gel texture. When strong, robust gels are required, the level of carrageenan / locust-bean gum / potassium chloride may be as high as 1.3%; levels of around 0.4% produce a weak, easily broken gel, which is preferred for some varieties. Low-acyl gellan gum has been successgully evaluated at plant scale in a number of canned pet foods and in dog-brawn. As for the carrageenan system, the gellan gum can be used alone, it is more commonly used with other hydrocolloids. For example, guar gum and xanthan gum are useful for providing syneresis control, while xanthan gum and locust-bean gum combinations or high-acyl gellan gum reduce gel brittleness. As a general rule of thumb, low-acyl gellan gum belends can replace k-carrgeenan blends in gelled pet foods at around half the use level. Thus, in a laboratory dog-brawn formulation, a blend of Kelcogel gellan gum, xanthan gum and locust-bean gum at 0.45% in combination with potato starch gave a similar product to a carrageenan / locust-bean gum blend at 1.0%, while in a prototype canned pet food 0.75% of a Kelcogel gellan gum . guar gum blene was able to replace carrageenan /locust-bean gum /potassium chloride at 1.3%.
The potential food uses of gellan gum have already been described and the major areas of application are listed in Table 2. Previously published food formulations indicate that low-cayl gellan gum is functional at very low use levels. These, however, were based on low-acyl, unclarified gellan gum. More recent studies have shown that Kelcogel food-grade gellan gum can be used in these formulations at around two-thirds of the low-cayl, unclarified gellan gun use level. In products using k-carrgeenan or agar as the primary gelling agent, Kelcogel can be used at around one-half to less than one-third of the use level to provide similar texture. The relative performance of Kelcogel and agar in selected Japanese foods is shown in Table3. The lower use levels of Kelcogel and the textural similarities of the end products are parent. The tokoroten noodles with Kelcogel also contain 0.4% of a 50:50 blend of xanthan gum and locust-bean gum to improve resilience. These examples illustrate that, while low- acyl gellan gum can be alone in many products, further textural variations may be achiebed by inclusion of xanthan gum / locust-bean gum combinations. One of the major advantages of gelatine is its so-called ‘melt-in-the-mouth’ characteristics. Low-acyl gellan gum does not melt in the mouth but, as already mentioned, the low use levels and the particular nature of the gel network result in outstanding flavour release. Thus, water dessert gels made with low-acyl gellan gum, although unlike the traditional gelatine gels in texture, are excellent appealing products that, upon consumption, resemble a beverage rather than a solid. The ability to produce such novel products suggests that gellan gum will be a valuable tool for the creation of new product concepts clarity of gellan gum is also a major advantage in certain applications.






